- Visibility 176 Views
- Downloads 16 Downloads
- DOI 10.18231/j.ijohd.2022.009
-
CrossMark
- Citation
Evaluation and comparison of streptococcus mutans and scardovia wiggsiae from the predentate to permanent dentition using polymerase chain reaction
- Author Details:
-
Sowjanya R *
-
Sapna Konde
-
Manisha Agarwal
-
Preetha Peethambar
-
Sahana N Prasad
Introduction
Dental caries is a complex and multifactorial chronic disease that is heavily influenced by numerous biomedical factors (e.g.: bacteria, host, genetics and diet) and by social determinants of health. The microbiota of dental caries is complex and includes acidogenic and aciduric organisms. Dental caries as an infectious, transmissible disease was first demonstrated by Keyes (1960).[1] A group of phenotypically similar bacteria, collectively known as the Mutans Streptococci (MS), has been indicated as the primary bacterial component involved in development of dental caries in humans.[2], [3] S. mutans was more sensitive to traditional methods of detection like selective agar plate culturing, S. mutans and Lactobacillus count test and calorimetric testing. However, with the advent of PCR, many novel presumed unculturable taxa in oral infections have been identified. Recent studies have revealed that S. mutans was detected in caries free populations and was not detected in all cases of childhood caries,[4], [5] suggesting that there could be other species that could have cariogenic potential. Molecular methods have identified a novel pathogen that may alter the current understanding of dental caries, which is Scardovia wiggsiae (S. wiggsiae). It is anaerobic, gram positive, non-motile, non-sporing bacillus with acidogenic and aciduric properties[6] in Bifidobacterium group, found to be associated with initiation and progression of dental caries. Scardovia wiggsiae has been associated with severe early childhood caries even in the absence of S. mutans.[7] There is a lacunae of studies showing the trend of these organisms across various dentition periods. In addition, no study states the time of initial acquisition of this micro-organism. Understanding the acquisition and colonization of these species may be essential for developing strategies to prevent dental caries. Hence this study was aimed to evaluate and compare the presence of S. mutans and S. wiggsiae from the predentate to permanent dentition period.
Materials and Methods
A randomised case-control study was conducted in Bangalore (Karnataka) among 80 children aged between 0 to 14 years. They were divided into 8 groups of 10 each. Clinical examination was performed by a single examiner using mouth mirror and straight probe under the natural light. Decayed, extracted and filled surfaces (def) in deciduous teeth & Decayed, Missing and Filled surfaces in permanent teeth were counted in each subject. (according to Gruebell. A.O in 1944).
Study design
Group 1 included children before the eruption of tooth.
Group 2 included children during the first tooth eruption.
Group 3 included children with no caries in primary dentition with def score 0.
Group 4 included children with early childhood caries in primary dentition with a def score of 5 or above
Group 5 included children with no caries in mixed dentition with deft and DMF score 0.
Group 6 included children with dental caries in mixed dentition with a deft and DMF score of 5 or above.
Group 7 included children with no caries in permanent dentition with DMF score 0.
Group 8 included children with dental caries in permanent dentition with DMF score of 5 or above.
Sample collection
The sample was collected by one of the following methods depending upon the age of the patient.
Swab method
Saliva sample was collected in cotton swabs for infants.
The swabs were swept across the facial, lingual, palatal and occlusal surfaces of the maxillary and mandibular arches.
The swabs were stored in MTM medium and stored at 20° Celsius until taken for DNA extraction.
Spitting method
The subject was asked to accumulate unstimulated saliva in the floor of the mouth and then spit into a sterile 50ml graduated container. 2ml of saliva was collected from each subject and was stored at 200c until taken for DNA extraction.
Microbial analysis using real-time polymerase chain reaction
Total genomic DNA from the saliva samples was isolated using N-Cetyl-N, N, N- trimethyl ammonium bromide (CTAB) method. The extracted DNA was qualified using Spectrophotometer (Sartorius) for standardizing the PCR.
The quantity of the isolated DNA was checked in UV-VIS spectrophotometer (Vivaspec Biophotometer, Germany). The primers for quantification analysis were designed using Perkin Elmer Primer.
Express® software. The Melting temperature (Tm) was calculated and the synthesized primers were purified by HPLC. The quantified DNA was used to detect the presence of S. mutans and S. wiggsiae using the specific primers below and primer optimization was done in a gradient PCR and found the annealing temperature as 60oC ([Table 1]). Quantification was performed in Applied Biosystems StepOne Real Time PCR (Foster City, CA). A standard curve with highest R2 value was constructed based on the values generated by the qPCR and the quantity of S. mutans and S. wiggsiae in each sample was calculated against the standard values.
Statistical analysis
Statistical Package for Social Sciences [SPSS] for Windows Version 22.0 Released 2013. Armonk, NY: IBM Corp., was performed for statistical analyses. Descriptive analysis includes expression of Scardovia wiggsiae and Streptococcus mutans levels using mean and standard deviation.
Results
The present study showed that both S. mutans and S. wiggsiae were present in the predentate group. However, S. mutans was significantly higher than S. wiggsiae in both the pre-dentate and first tooth eruption group at P<0.001 ([Table 2])
S. mutans was significantly higher in control groups than in experimental groups, whereas S. wiggsiae were significantly higher in experimental groups than the control groups ([Table 3], [Figure 1]). There was a steady increase in both the organisms from predentate to mixed dentition period followed by a decrease in permanent dentition ([Table 3], [Figure 1] )
|
Primer |
Sequence (5' - 3') |
|
|
S. mutans |
FP |
GCCTACAGCTCAGAGATGCTATTCT |
|
RP |
GCCATACACCACTCATGAATTGA |
|
|
S. wiggsiae |
FP |
GTGGACTTTATGAATAAGC |
|
RP |
CTACCGTTAAGCAGTAAG |
|
Group |
Organism |
N |
Mean |
SD |
Mean Diff |
P-Value |
|
Pre-dentate |
S. mutans |
10 |
418.10 |
320.83 |
364.80 |
0.001* |
|
S. wiggsiae |
10 |
116.30 |
103.05 |
|||
|
1st Tooth Eruption |
S. mutans |
10 |
1702.10 |
652.99 |
1245.70 |
<0.001* |
|
S. wiggsiae |
10 |
456.40 |
133.11 |
|
Dentition |
Group |
Organism |
N |
Mean |
SD |
Mean Diff |
P-Value |
|
Primary |
Control |
S. mutans |
10 |
4013.10 |
1446.39 |
2907.50 |
<0.001* |
|
S. wiggsiae |
10 |
1105.60 |
521.49 |
||||
|
Test |
S. mutans |
10 |
3519.50 |
2488.59 |
-6939.60 |
<0.001* |
|
|
S. wiggsiae |
10 |
10459.10 |
2670.35 |
||||
|
Mixed |
Control |
S. mutans |
10 |
5107.30 |
1033.88 |
3640.70 |
<0.001* |
|
S. wiggsiae |
10 |
1466.60 |
377.98 |
||||
|
Test |
S. mutans |
10 |
2698.60 |
1312.79 |
-8984.00 |
<0.001* |
|
|
S. wiggsiae |
10 |
11682.60 |
4227.02 |
||||
|
Permanent |
Control |
S. mutans |
10 |
2418.80 |
723.28 |
1339.60 |
<0.001* |
|
S. wiggsiae |
10 |
1079.20 |
155.83 |
||||
|
Test |
S. mutans |
10 |
1396.00 |
667.40 |
-4466.00 |
<0.001* |
|
|
S. wiggsiae |
10 |
5862.00 |
2581.16 |
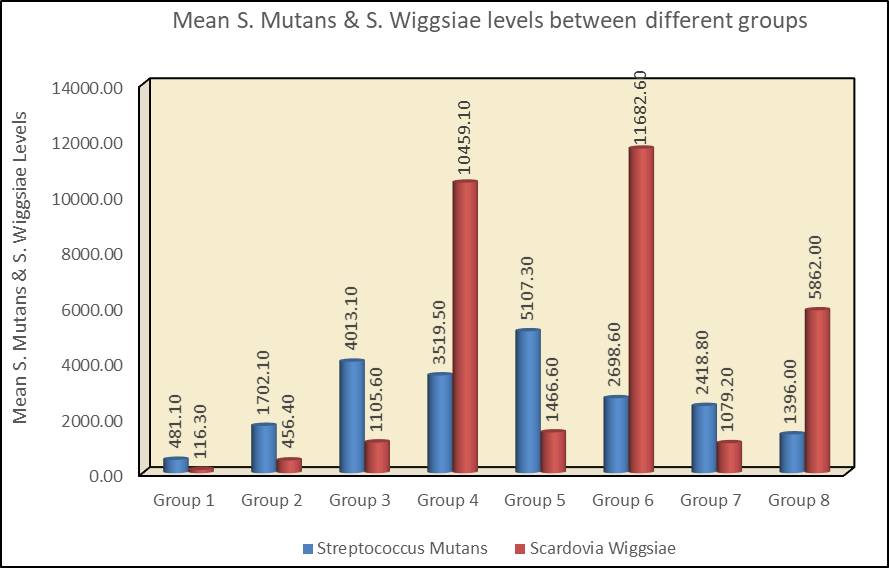
Discussion
Dental caries has been included in a group of conditions like cancer or diabetes which has multifactorial etiology.[8] The factors that can vary prevalence pattern and severity of dental caries are age, sex, race, socio-economic status, geographical location, food habit and habits of oral hygiene.[9] The three major hypothesis that explains the etiology of dental caries are: the specific, the non-specific and the ecological plaque hypothesis.[10], [11], [12] The specific plaque hypothesis states that only few specific species are actively involved in the disease like streptococcus mutans and streptococcus sobrinus, and on the other hand the nonspecific plaque hypothesis proposes that the dental caries is a result of overall activity of entire plaque microflora which contains numerous bacterial species.[11] The ecological plaque hypothesis suggests that dental caries occurs when there is a shift in the balance of resident microflora which is driven by changes in local environmental conditions.[12]
Although Streptococcus mutans was isolated from carious lesion by James Kilian Clarke in 1924, the genuine interest in this bacterium was generated in 1960s when researchers began studying dental caries.[13] Ability to initiate and maintain microbial growth, continue producing acid at low pH values, rapid metabolism of sugars to lactic and other organic acids, ability to attain the critical pH for enamel demineralization more rapidly than other common plaque bacteria and ability to produce Intracellular Poly-Saccharides (IPSs) as glycogen, are some of the factors responsible for cariogenicity of Streptococcus mutans.[14], [15] Milgrom et al in 2000, found that children with increased levels of S. mutans were five times more prone to have dental caries than children with lower levels of S. mutans.[16] However, today S. mutans and its involvement in dental caries is debatable. Recent studies have shown the association of S. wiggsiae in dental caries in the absence of S. mutans.
Results of the present study showed both S. mutans and S. wiggsiae were present in all the study groups ([Table 2], [Table 3] and [Figure 1]). S. mutans was significantly higher than S. wiggsiae in the predentate period. Though an early study did not report the presence of MS in young children before tooth eruption,[17] Milgrom et al. (2000) stated that 25% of 6-month-old infant had MS colonization,[16] however Wan et al (2001) found that 50% of 6-month-old predentate children had MS colonization.[18] The present study showed that all children in the predentate group had both the organisms. The wide range of MS prevalence found among investigations may be due to differences in the sensitivity of MS detection of the various media used.[19] The present study showed that S. wiggsiae was present in the predentate period as early as 1 month. Xu et al in 2015 showed that phyla Actinobacteria had colonized all neonate’s mouths by 3 or 4 days after delivery. Bifidobacterium belongs to phyla Actinobacteria and Scardovia species belong to genus Bifidobacterium.[20] However further studies are required to substantiate the time of initial acquisition of S. wiggsiae.
Although S. mutans are present in the predentate period, it favors hard, non-desquamating surface for establishment of permanent colonies. Eruption of tooth provides new binding site and new ecological events take place in the oral environment, Streptococcus mutans accelerates its colonization at this stage,[21] S. wiggsiae too had increased in number with the eruption of tooth but it was significantly lesser than S. mutans.
Present study also showed the trend of both the organisms across different dentition period in both caries-free and caries associated children, which revealed that both organisms increased from predentate to mixed dentition period followed by a decrease in permanent dentition ([Table 3], [Figure 1]). During the mixed-dentition phase, the oral ecosystem undergoes several changes related to the eruption of permanent teeth and onset of puberty.[22] Adolescence is characterized by major hormonal changes, which is associated with nutritional enrichment of the oral environment. This event results in increase of some groups of oral microorganisms, including gram-negative anaerobes and spirochetes.[23] However, the count of both the organisms decreased in permanent dentition, this is because, as per ecological plaque hypothesis the changes in ecological factors requires different bacterial qualities and stimulates alterations in composition of the bacteria.[4] In addition, there is improvement in oral hygiene practice in older children as compared to the younger ones, as tooth brushing skills improve with age.[24]
The present study showed that S. mutans counts was significantly higher than S. wiggsiae in control groups across the various dentition period ([Table 3], [Figure 1]) whereas, S. wiggsiae was significantly higher than S. mutans in experimental groups ([Table 3], [Figure 1]). Tanner et al in 2011 stated that S. wiggsiae was associated with severe ECC irrespective of the presence or absence of S. mutans.[7] Vacharaksa et al in 2015, stated that the colonization with S. wiggsiae was related to ECC; and many caries-free children had a detectable level of S. mutans as part of their commensals.[25] Hence, presence of S. mutans alone may not precisely represent caries status. Present study is also in accordance with studies done by Unsal G et al 2017,[26] Pretika Chandna et al 2018[27] and Sucheta Prabhu Matondka et al 2019[28] which concluded a positive association of S. wiggsiae with ECC.
Mello de Matos- 2017 through dual-species biofilm model containing bifidobacteria and Streptococcus mutans revealed that Bifidobacteria in combination with S. mutans not only produced significant amount of acids but also accentuated acidogenicity of biofilm, as S. mutans metabolizes carbon sources at a higher rate, producing acids faster than bifidobacteria and lactobacilli. Both bifidobacteria and lactobacilli favors lower pH to produce acids, thus S. mutans promotes a favorable environment for these species to thrive. Bifidobacteria metabolizes raffinose to a higher extent than L. acidophilus and S. mutans. This is a significant finding as bifidobacteria do not require the consumption of sweets or snacks for acid production, since raffinose is present naturally in healthy foods.[29] In addition, S. wiggsiae primarily being an anaerobic bacterium can survive and continue to produce acid under more mature biofilm with low oxygen unlike S. mutans which is a facultative anaerobic bacterium.[30]
Mai Kameda et al in 2020 showed that S. wiggsiae produces acetic acid as their major end product from a unique metabolic pathway known as F6PPK (fructose-6- phosphate pathway) shunt, whereas S. mutans and other caries associated bacteria produces lactic acid as their main end product.[30] Hoppenbrouwers et al in 1998 stated that when compared to lactic acid, acetic acid is non-ionized in low pH environments, and penetrates the enamel faster and decalcify it than lactic acid.[31] Hence it may be concluded that S. wiggsiae which produces acetic acid is involved in caries progression than S. mutans which produces lactic acid which aids in lowering the pH and favoring other organisms like S. wiggsiae to thrive.
Through the bulk of evidence, it can be concluded that the relation of S. mutans and dental caries can be associative but not causative.[32] Acid production by dental plaque is not solely dependent upon the presence of mutans streptococci; caries can occur in the absence of these species and their presence does not necessarily indicate caries activity. Other oral bacterias like non-mutans streptococci, Actinomyces spp. and Bifidobacterium spp., are acidogenic and aciduric and they outnumber mutans streptococci in dental caries.[33]
The present study showed that S. wiggsiae was significantly higher in experimental groups whereas S. mutans was higher in controls suggesting that S. wiggsiae has a major role in development and progression of dental caries and S. mutans could be the commensal.
Conclusion
S. mutans and S. wiggsiae were present in predentate period and counts of both the organisms increased from predentate to mixed dentition period and decreased in permanent dentition period. S. wiggsiae was significantly higher in experimental groups suggesting a major role in development of dental caries whereas, S. mutans was higher in control groups indicating that it could be a commensal in the oral cavity.
Source of Funding
None.
Conflict of Interest
The authors declare no conflict of interest.
References
- P Keyes. The infectious and transmissible nature of experimental dental caries. Arch Oral Biol 1960. [Google Scholar]
- RJ Gibbons, JV Houte. Dental Caries. Ann Rev Med 1975. [Google Scholar]
- WJ Loesche, J Rowan, LH Straffon, PJ Loos. Association of Streptococcus mutans with Human Dental Decay. Infect Immun 1975. [Google Scholar]
- JA Aas, AL Griffen, SR Dardis, AM Lee, I Olsen, FE Dewhirst. Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adults. J Clin Microbiol 2008. [Google Scholar]
- WJ Loesche, J Rowan, LH Straffon, PJ Loos. Association of Streptococcus mutans with Human Dental Decay. Infect Immun 1975. [Google Scholar]
- J Downes, M Mantzourani, D Beighton, S Hooper, MJ Wilson, A Nicholson. Scardovia wiggsiae sp. nov., isolated from the human oral cavity and clinical material, and emended descriptions of the genus Scardovia and Scardovia inopinata. Int J Syst Evol Microbiol 2011. [Google Scholar]
- ACR Tanner, JMJ Mathney, RL Kent, NI Chalmers, CV Hughes, CY Loo. Cultivable Anaerobic Microbiota of Severe Early Childhood Caries. J Clin Microbiol 2011. [Google Scholar]
- O Fejerskov. Changing Paradigms in Concepts on Dental Caries: Consequences for Oral Health Care. Caries Res 2004. [Google Scholar]
- PE Petersen, D Bourgeois, H Ogawa, S Estupinan-Day, C Ndiaye. The global burden of oral diseases and risks to oral health. Bull World Health Organ 2005. [Google Scholar]
- WJ Loesche. The specific plaque hypothesis and the antimicrobial treatment of periodontal disease. Dent Update 1992. [Google Scholar]
- E Theilade. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol 1986. [Google Scholar]
- P Marsh. Microbial Ecology of Dental Plaque and its Significance in Health and Disease. Advances in Dental Research 1994. [Google Scholar]
- DC Melton, KV Krell, MW Fuller. Anatomical and histological features of C-shaped canals in mandibular second molars. J Endod 1991. [Google Scholar]
- P Marsh, M Martin, M Lewis. Plaque mediated diseases - dental caries and periodontal diseases. Oral microbiology 2009. [Google Scholar]
- J Bagg, TW Macfarlane, IR Poxton. . Dental caries in essentials of microbiology for dental students 2006. [Google Scholar]
- P Milgrom, CA Riedy, P Weinstein, ACR Tanner, L Manibusan, J Bruss. Dental caries and its relationship to bacterial infection, hypoplasia, diet, and oral hygiene in 6- to 36-month-old children. Community Dent Oral Epidemiol 2000. [Google Scholar]
- P Caufield, G Cutter, A Dasanayake. Initial Acquisition of Mutans Streptococci by Infants: Evidence for a Discrete Window of Infectivity. J Dent Res 1993. [Google Scholar]
- A Wan, W Seow, D Purdie, P Bird, L Walsh, D Tudehope. Oral Colonization of Streptococcus mutans in Six-month-old Predentate Infants. J Dent Res 2001. [Google Scholar]
- KA Plonka, ML Pukallus, AG Barnett, LJ Walsh, TH Holcombe, WK Seow. Mutans Streptococci and Lactobacilli Colonization in Predentate Children from the Neonatal Period to Seven Months of Age. Caries Res 2012. [Google Scholar]
- X Xu, J He, J Xue, Y Wang, K Li, K Zhang. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol 2014. [Google Scholar]
- J Xiao, KA Fiscella, SR Gill. Oral microbiome: possible harbinger for children’s health. Int J Oral Sci 2020. [Google Scholar] [Crossref]
- AMM Kaan, D Kahharova, E Zaura. Acquisition and establishment of the oral microbiota. Periodontology 2000. [Google Scholar]
- H Jenkinson, R Lamont, R Lamont, RA Burne, MS Lantz, DJ Leblanc. Oral Microbial Ecology. Oral microbiology and immunology 2006. [Google Scholar]
- P Pujar, VV Subbareddy. Evaluation of the tooth brushing skills in children aged 6-12 years. Eur Arch Paediatr Dent 2013. [Google Scholar]
- A Vacharaksa, P Suvansopee, N Opaswanich, W Sukarawan. PCR detection of Scardovia wiggsiae in combination with Streptococcus mutans for early childhood caries-risk prediction. Eur J Oral Sci 2015. [Google Scholar]
- G Unsal, N Topcuoglu, I Ulukapi, G Kulekci, O Aktoren. Scardovia Wiggsiae and the Other Microorganisms in Severe Early Childhood Caries. J Dent Oral Care Med 2017. [Google Scholar]
- P Chandna, N Srivastava, A Sharma, V Sharma, N Gupta, V Adlakha. Isolation of Scardovia wiggsiae using real-time polymerase chain reaction from the saliva of children with early childhood caries. J Indian Soc Pedod Prev Dent 2018. [Google Scholar]
- SP Matondkar, C Yavagal, M Kugaji, KG Bhat. Quantitative assessment of Scardovia wiggsiae from dental plaque samples of children suffering from severe early childhood caries and caries free children. Anaerobe 2020. [Google Scholar] [Crossref]
- BM De Matos, FL Brighenti, T Do, D Beighton, CY Koga-Ito. Acidogenicity of dual-species biofilms of bifidobacteria and Streptococcus mutans. Clin Oral Investig 2017. [Google Scholar]
- M Kameda, Y Abiko, J Washio, ACR Tanner, C A Kressirer, I Mizoguchi. Sugar Metabolism of Scardovia wiggsiae, a Novel Caries-Associated Bacterium. Front Microbiol 2020. [Google Scholar] [Crossref]
- P Hoppenbrouwers, F Driessens. The Effect of Lactic and Acetic Acid on the Formation of Artificial Caries Lesions. J Dent Res 1988. [Google Scholar]
- W Sims. Streptococcus mutans and vaccines for dental caries: a personal commentary and critique. Community Dent Health 1985. [Google Scholar]
- D Beighton. The complex oral microflora of high-risk individuals and groups and its role in the caries process. Community Dent Oral Epidemiol 2005. [Google Scholar]
How to Cite This Article
Vancouver
R S, Konde S, Agarwal M, Peethambar P, Prasad SN. Evaluation and comparison of streptococcus mutans and scardovia wiggsiae from the predentate to permanent dentition using polymerase chain reaction [Internet]. Int J Oral Health Dent. 2025 [cited 2025 Sep 07];8(1):35-40. Available from: https://doi.org/10.18231/j.ijohd.2022.009
APA
R, S., Konde, S., Agarwal, M., Peethambar, P., Prasad, S. N. (2025). Evaluation and comparison of streptococcus mutans and scardovia wiggsiae from the predentate to permanent dentition using polymerase chain reaction. Int J Oral Health Dent, 8(1), 35-40. https://doi.org/10.18231/j.ijohd.2022.009
MLA
R, Sowjanya, Konde, Sapna, Agarwal, Manisha, Peethambar, Preetha, Prasad, Sahana N. "Evaluation and comparison of streptococcus mutans and scardovia wiggsiae from the predentate to permanent dentition using polymerase chain reaction." Int J Oral Health Dent, vol. 8, no. 1, 2025, pp. 35-40. https://doi.org/10.18231/j.ijohd.2022.009
Chicago
R, S., Konde, S., Agarwal, M., Peethambar, P., Prasad, S. N.. "Evaluation and comparison of streptococcus mutans and scardovia wiggsiae from the predentate to permanent dentition using polymerase chain reaction." Int J Oral Health Dent 8, no. 1 (2025): 35-40. https://doi.org/10.18231/j.ijohd.2022.009
